Following the same method as above we can write. Write what each symbol in the ideal gas law represents the unit in which it is measured and the abbreviation of the unit.

Combined Gas Law Overview Calculations Expii
If any two of the four factors are.

. I will use 273 K zero degrees Celsius for standard temperature. Complete the table about the ideal gas law. The reservoir pressure and.
R rate of diffusion or effusion. T 0 0 C 273 0 K. M molar mass.
V 2 V 1 T 2 T 1 V 2 7485225 32315 V 2 2316 mL Gay-Lussac Law Gay-Lussac law gives the relationship between temperature and pressure at constant volume. Ideal Gases and Real Gasespages 428429 9. We have talked about four variables that affect the behavior of gases.
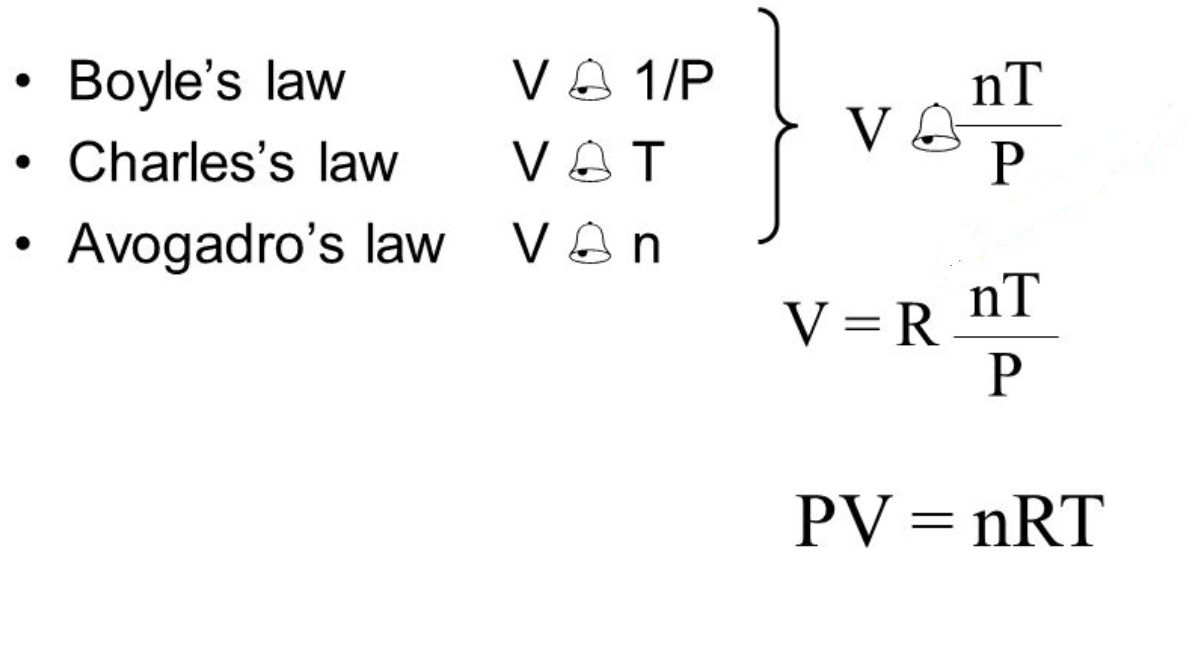
Pressure P volume V number of moles of gas n and temperature T. Must follow the Kinetic Molecular Theory of Gases. The Ideal Gas Law may be expressed in SI units where pressure is in pascals volume is in cubic meters N.
PV stays constant as pressure increases with moles and temperature held constant as expected by the ideal gas law PV nRT. P is the pressure V is the volume N is the number of moles of gas R is the universal gas constant and T is the absolute temperature. 53 Covalent Bonds 54 Bonding in Metals.
The number of particles or molecules is proportional to the number of moles n the constant of proportionality being Avogadros number N0. If V is the volume and T is the temperature of a gas at some constant pressure then V T or VT constant. Check with your teacher on this point.
Combined gas law formula. The rates of two gases can be compared to each other using the formula. Mathematical representation of the law is given in the table.
The temperature T is absolute temperature. The Kelvin temperature of a gas is directly proportional to its kinetic energy. PV constant P 1 V 1 P 2 V 2 P 3 V 3 constant Where P is the pressure of a gas V is the volume of gas Charless Law.
P 1 atm or its equivalents. Amount in moles of gas molecules. Combined these form the Ideal Gas Law equation.
Ideal gas behavior is assumed at the standard condition used for natural gas metering. V volume usually in liters n number of particles of gas. P is the pressure V is the volume N is the number of moles of gas R is the universal gas constant and T is the absolute temperature.
The VdW equation basically incorporates the effect of gas molecule volume and intermolecular forces into the ideal gas equation. For example 25 C 298 K because 25 273 298. Often the value of 273 is used instead of 27315.
If the total volume V contains n moles of gas then only v V n appears in the equation of state. R M 12 constant. Hence μ.
As mentioned in the previous modules of this chapter however the behavior of a gas is often non-ideal meaning that the observed. The Ideal Gas Law may be expressed as. 1 atm 1 atmosphere 760 torr 760 mm 76 m Hg.
Therefore the perfect gas equation is written as PV μ RT. Where P pressure V volume N number of gas molecules k Boltzmann constant 138110 23 JK 1 in SI units and T temperature K. The force exerted by gas molecules as they strike a given surface.
When the amount of gas and pressure remain constant find the new volume of Carbon dioxide in the pump if the temperature is increased to 750 o C. V₁T₁ V₂T₂ Gay Lussacs or Regnaults Law This law states that at constant volume V the pressure P of a given mass of a gas is directly proportional to its absolute temperature T. Temperature is always in Kelvin.
P absolute pressure in atmospheres. Amost all examples done by the ChemTeam will use 273. Volume increases linearly as temperature increases with moles and pressure held constant as expected by the ideal gas law V nRPT.
A term used to describe the relationship between two variables whose graph is a straight line passing through point 00 the origin. By choosing the Kelvin scale for absolute temperature we get R 8314 J mol 1 K 1. The factors we must consider to study gas behavior are.
Thus far the ideal gas law PV nRT has been applied to a variety of different types of problems ranging from reaction stoichiometry and empirical and molecular formula problems to determining the density and molar mass of a gas. At absolute zero 0 K molecules stop moving entirely the gas is as cold as anything can get. Grahams law atates the rate of diffusion or effusion for a gas is inversely proportional to the square root of the molar mass of the gas.
The infrastructure model is a mathematical representation of the actual system. Compressibility factor Z varies as PVRT increases as expected of a real gas. The four gas variables are.
The universal gas constant R is a number that satisfies the proportionalities of the pressure-volume-temperature relationship. A measure of how fast gas molecules move kinetic energy. Thus at constant temperature and pressure the volume of a gas is proportional to the number of moles.
Chapter 14 The Behavior of Gases153 7. We can use a number of different equations to model the behavior of real gases but one of the simplest is the van der Waals VdW equation. 3 where μ is the number of moles and R N A.
According to Boyles law the volume of the gas is inversely related to pressure when the amount of gas is fixed at a constant temperature. Another formulation of the ideal gas law can be. Standard Temperature and Pressure STP or Standard Conditions SC.
Thus far the ideal gas law PV nRT has been applied to a variety of different types of problems ranging from reaction stoichiometry and empirical and molecular formula problems to determining the density and molar mass of a gas. K B is a universal constant. K Boltzmanns constant 13810 23 JK 1 T temperature in Kelvin.
The space occupied by gas molecules. 33 Gas Behavior 34 Graphing Gas Behavior. Barton in Proceedings of the 1st Annual Gas Processing Symposium 2009 4 The Infrastructure Model.
Combined these form the Ideal Gas Law equation. When would you use the ideal gas law instead of the combined gas law. Developed during the mid-19th century by several physicists including the Austrian Ludwig Boltzmann 18441906 the German Rudolf Clausius 18221888 and the Englishman James Clerk Maxwell 18311879 who is also known for his contributions to.
The kinetic molecular theory of gases explains the laws that describe the behavior of gases. 51 Atoms Bonding 52 Ionic Bonds. The trunkline network wells and facilities.
The universal gas constant R is a number that satisfies the proportionalities of the pressure-volume-temperature relationship.

Ideal Gas Law Formula And Examples

Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry
0 Comments